Effects of Butea superba on reproductive systems of rats
Abstract (Butea superba on reproductive systems)
The effects of Butea Superba on the reproductive system in male Wistar rats were investigated. The animals were fed daily with the powdered crude drug suspended in distilled water by a gastric tube at the dose of 2, 25, 250 and 1250 mg/kg body weight for 8 weeks. Rats fed with 1 ml of distilled water were used as a negative control. The weights of all vital organs in all treated groups were not different from the control. The percentage weight ratios of body weights of seminal vesicles and prostate glands were not different from the control, except that the testis of the group fed with 1250 mg/kg was significantly different from the control and the other treated groups. In addition, the sperm counts in this group showed about 16% more than the control group. Hematology as well as the liver and kidney function of all treated groups showed no difference from the control. B. superba, drug at 250 mg/kg which was 100 times more than the Thai FDA recommended dose for humans appeared to be safe in rats. The crude drug has demonstrated an increase tendency on testis weight and sperm counts in rat. The information from the present study can be used to explain the Thai folklore application of this plant in Thailand. © 2006 Elsevier B.V. All rights reserved.
Keywords: Butea superba; Male reproductive systems; Thai folklore medicines
Introduction
Butea Superba, Roxb (Leguminosae) is a big climber plant, called “Kwao Krua Dang” in Thailand. Its underground tuberous roots have been used in Thai folklore medicines for over 100 years.
It has been used for the rejuvenation and treatment of impotence inmen.When the skin of the tuberous rootswas peeled off, red exudate with bloody appearance can be seen. The main constituent found in B. superba is butenin. However, when this compound was injected subcutaneously in rat at 360 mg/kg, body weight (b.w.), it caused death from failure of the cardiovascular system. Toxicity of butenin in mice has been previously reported [3]. Presently, various herbal formulations containing crude drug of B. superba are available in the Thai market. Thai FDA has been trying to onitor for consumer protection of these herbal products as safe traditional herbal recipes. Several studies have been reported on this aspect . The Thai FDA has recommended a safe daily dose of 100 mg/day or 0.2 mg/100 g b.w. for a 50 kg body weight person. The aim of this study is to investigate the effects of B. superba drug at the dose recommended by Thai FDA and about 10, 100 and 600 times higher than this recommended dose on the reproductive systems of maleWistar rats. The information from this study can be used to explain and consider the proper application of this plant.
Experimental
Plant
B. superba tuberous roots, collected in February 2002 from Chiang Mai Province in Thailand were authenticated by the Botanist of Faculty of Pharmacy, Chiang Mai University, Thailand. A voucher specimen (PCRNC-BS-02-2002) was deposited in the Pharmaceutical-Cosmetic RawMaterials and Natural Products Research and Development Center (PCRNC), Institute for Science and Technology Research and Development (IST), Chiang Mai University, Thailand.
Preparation of the tuberous roots suspension
The roots were washed and the skin was peeled off. The pulp of the root was cut into thin pieces and dried at 50±1 °C for 24 h. The dried samples were ground, passed through a sieve (No. 80) and suspended in distilled water (0.5, 5, 50 and 250 mg/ml).
Animals
Fifty male Wistar rats weighing 200–250 g were purchased from the Institute of Animal Facilities, Mahidol University, Bangkok, Thailand. The animals were housed under standard conditions at 25±2 °C and fed with standard pellet diet and tap water. All experiments on the animals were conducted under surveillance of the Ethic Committee of the Natural Products for Thai Traditional Medicine Research Unit, Faculty of Pharmacy, Institute for Science and Technology Research and Development, Chiang Mai University in Thailand.
Effects of drug suspension on rat reproductive systems
Rats were divided into 5 groups with 10 animals each. The first group was fed with 1 ml of distilled water and used as the negative control. Groups 2–5 were treated by a gastric tube with the drug suspension at the doses of 2, 25, 250 and 1250 mg/kg/day for 8 weeks. The body weights of the animals were recorded every week. After 8 weeks, the animals were killed. The vital organs (heart, spleen, liver, lung and kidney) and the reproductive organs (testis, seminal vesicles and prostate glands) were examined and weighed. The numbers of the sperms in the epididymis were counted. The blood samples were collected and the sera were assayed for hematology (hemoglobin, hematocrit, WBC, neutrophil, eosinophil, lymphocyte, monocyte and platelets), and liver/kidney function (BUN, creatinine, AST, ALT and alkaline phosphatase).

Fig. 1. Effect of the B. superba drug suspension given by oral route to rats for 8 weeks on body weight gain. Control: 1 ml of distilled water/day; BSa: 2 mg/kg; BSb: 25 mg/kg; BSc: 250 mg/kg; BSd: 1250 mg/kg.
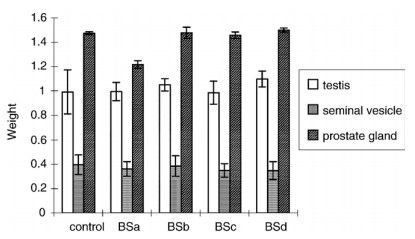
Fig. 2. Effect of the B. superba drug suspension given by oral route to rats for 8 weeks on reproductive organ/body weight gain. Control: 1 ml of distilled water/day; BSa: 2 mg/kg; BSb: 25 mg/kg; BSc: 250 mg/kg; BSd: 1250 mg/kg.
Statistical analysis
All experiments were performed in triplicate and the results were expressed as mean±SEM. Statistical significance was analyzed using Student’s t test. Pb0.05 was considered significant.
Results
There is no difference in the body weight gain between the treated groups and the control group after 8 weeks of treatment (Fig. 1). Also, the weights of the vital organs of the treated group were not different from the control group (data not shown). The percentage weight ratios of the reproductive organs (testis, seminal vesicle and prostate glands) to body weights of only the weight ratio of the testis were increased in animals treated with the highest dose of 1250 mg/kg (Fig. 2). All treated groups gave higher sperm counts of about 16% more than the control group without dose–response relationship (Table 1). For blood analysis, no difference between the treatment and the control groups in hemoglobin, hematocrit, WBC, neutrophil, eosinophil, basophil, lymphocytes, monocytes and platelet counts was shown (data not shown). All treated groups also indicated no difference from the control group in liver and kidney function of BUN, creatinine, AST, ALT and alkaline phosphatase (data not shown).
Discussion and conclusions
During the 8-week treatment, body weights and weights of all vital organs of the animals treated with B. Superba drug at all doses showed no difference from the control animals fed with distilled water. The group treated with the highest dose of B. superba crude drug 1250 mg/kg showed significant (Pb0.05) higher percentage weight ratio to body weight of testis than the control and the other treated groups. B. superba which has been claimed by the Thai northern traditional folklore as treatment for impotency in men may contain compounds which had androgenic activity [1,2]. The androgenic compounds can increase the release of GnRH (gonadotropic hormone) from the hypothalamus thereby increasing the release of male sex hormone. This hormone is responsible for the growth of sertoli cells and leydig cells that can augment the size of the testis [5,6]. All treated groups in this study have also demonstrated higher sperm numbers than the control group. Compounds in B. superba may have the similar activity to FSH (follicle stimulating hormone) or testosterone that can increase the production of sperms [7].
The information from this study can be used to explain the Thai folklore application of this plant which has been used to increase sexuality in men.
Table 1
Effect of the B. superba drug suspension given by oral route to rats for 8 weeks on sperm counts

References
Butea superba on reproductive systems
[1] Soonthorn L. Herbal recipe of tuberous Kwao Krua. Chiang Mai: Uppatipong Publisher; 1931. p. 15.
[2] Manosroi A, Saowakon S, Manosroi J. Kwao Krua. The Second National Pharmaceutial Biotechnology Seminar. Institute for Science and Technology Research and Development Center. Chiang Mai, Thailand: Chiang Mai University; 2000. p. 71.
[3] Boonyaprapat N, Chokchaijarunporn A. Folk medicinal plants (Thai). Bangkok: Prachachon Ltd Publisher; 1996. p. 308.
[4] Kwao Krua, editor. Hope of Thai medicinal plants, vol. 14. Update; 1999. p. 40.
[5] Lincoln GA. Br Med Bull 1979;35:167.
[6] Turner CD, Bagnara TJ. General endocrinology. 6th ed. Philadelphia, PA: Saunders Co.; 1976. p. 596.
[7] Balin H, Glasser S. Reproductive biology. Amsterdam: Van Corcum Ltd.; 1972. p. 973.
Aranya Manosroi, Kanokporn Sanphet, Suda Saowakon, Salika Aritajat, Jiradej Manosroi
Natural Products for Thai Traditional Medicine Research Unit, Pharmaceutical-Cosmetic Raw Materials and Natural Products Research and Development Center (PCRNC), Institute for Science and Technology Research and Development, Chiang Mai University, Chiang Mai 50200, Thailand Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200, Thailand Reproductive Physiology Research Unit, Department of Biology, Faculty of Sciences, Chiang Mai University, Chiang Mai 50200, Thailand
Received 4 March 2005; accepted 24 May 2006
Available online 6 July 2006
Butea superba on reproductive systems
Should you require any other information, please contact customer service email cs@orientalheritageherbalists.com






