Estrogen-like activities and cytotoxicity effects of Thai herbal medicines as natural ingredients in anti-aging
Estrogen like activities
The objective of the study was to search for the appropriate herbal extracts by comparative analysis of their estrogenic and cytotoxic activities. Some potentially estrogenic activity of herbal extracts in the management of female disorder symptoms was investigated by E-screen assay. The extracts having a promising activity were further evaluated in vitro for their cytotoxic activity. Of 13 herbal extracts tested, 10 showed interesting estrogenic properties. These were Pueraria candollei var mirifica, Linum usitatissimum, Glycine max, Curcuma aeruginosa, Cissus quadrangularis, Tadehagi godefroyanum, Curcuma comosa, Butea superba, Trigonella foenum-graecum and Punica granatum. The proliferative activity of those extracts could be completely inhibited by the addition of an estrogen receptor antagonist. The extract of T. foenum-graecum exhibited the strongest cytotoxicity on mouse fibroblast cells (cell viability <80% at 100 µg/ml) while a growth-promoting effect could be observed for P. candollei var mirifica, C. aeruginosa, C. quadrangularis and C. comosa. Pre-treatment of those 10 extracts to the mouse fibroblast cells prior to the addition of H2O2 reduced the apoptotic cells as well as increased the percentage of cell survival. Nine (9) extracts (not T. foenum-graecum) were selected as potentially active ingredients for the treatment of skin-ageing in post-menopausal women.
Key words: Phytoestrogen, MCF-7 cells, cytotoxicity, traditional Thai medicine, anti-skin-ageing product.
INTRODUCTION
In recent years, phytoestrogens have been found to be beneficial to skin ageing in post-menopausal women. These efficacies are through the exertion of estrogen-mimicking effects via their structural similarity to estrogens. The in vitro study of Tomaszewski et al. (2003) showed that phytoestrogens such as daidzein increased the proliferation of skin fibroblasts similar to that of 17β-estradiol (E2) but was lower in efficacy. An in vivo study reported that Bifidobacterium-fermented soy milk extract could increase skin elasticity (Miyazaki et al., 2004). Moreover, topical application of isoflavones containing emulsions improved the number of dermal papillae per area after 2 weeks of another in vivo study (Südel et al., 2005), suggesting that the use of phytoestrogens as an active ingredient in cosmetic preparation may have the same efficacy as E2 against skin ageing in post-menopausal women. Numerous research work showed that several herbs contain phytoestrogens, for example Glycine max (Lee et al., 2011), Linum usitatissimum (Attoumbré et al., 2011) and Pueraria candollei var mirifica (Boonchird et al., 2010). However, most studies have determined the estrogenic activity of individual pure compounds or herb species. Comparative investigation of estrogenic activity of most herbs containing phytoestrogens has not been elucidated to select the most appropriate one to be used as compositions in anti-skin-ageing products. The present study aimed to search for the appropriate herbal extracts by comparative analysis of their estrogenic and cytotoxic activities. Herbal extracts with good estrogen-like effects and low degrees of cytotoxicity were to be used as active ingredients in anti-skin-ageing preparations. To achieve this, crude ethanolic extracts of 13 herbal medicines were investigated for their biological activities. The criteria for selecting the suitable extracts were good estrogen-like effect, low cytotoxicity, evident data reported, and information from traditional herbal medicine practitioners. Moreover, other biological activities associated with the anti-ageing effect, such as protective effects against oxidative stress were also compared.
MATERIALS AND METHODS
Reagents
E2, ICI 182780 and 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were obtained from Sigma-Aldrich Inc. (St. Louis, MO, USA); Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum albumin, trypsin/ ethylenediaminetetraacetic acid (EDTA), Pen/Strep (10,000 units/ml penicillin and 10,000 µg/ml streptomycin), from Gibco Invitrogen Corporation (NY, USA). All chemicals were of analytical grade and obtained from commercial sources.
Herbal extraction
Thirteen herbal medicines were identified and their voucher specimens were deposited at the herbarium of the Faculty of Pharmaceutical Sciences, Ubon Ratchathani University, Thailand. The herbal powders were extracted by maceration in ethyl alcohol. The macerated mixtures were pooled and filtered through a membrane filter (Whatman® No. 4, USA). Solvents were removed under the vacuum of a rotary evaporator (BÜCHI, Flawil, Switzerland) at 45±1°C. The resulting extracts were freeze-dried and kept at -20°C until used.
Evaluation of estrogen-like effect
The estrogen receptor positive human mammary adenocarcinoma (MCF-7) cells were routinely maintained in DMEM containing 10% heat-inactivated fetal bovine serum (FBS), supplemented with 1% Penicillin/Streptomycin. The cells were maintained in a cell incubator in a humidified atmosphere of 5% CO2 and 95% air at 37°C. The MCF-7 cells were harvested and seeded into a 96-well plate at a density of 1×103 cells/well in DMEM supplemented with 10% FBS. The cells were allowed to attach for 48 h. The culture medium was removed and washed with phosphate buffer saline solution (PBS). Then, the medium was changed to phenol red-free DMEM supplemented with 7% dextran-coated charcoal FBS and the cells were incubated for another 48 h. After pre-treatment, the cells were treated with herbal extract solutions. The experimental cells were re-treated with herbal extract every 48 h. The cell proliferation was measured on the sixth day following the treatment (cell proliferation of positive control wells reached 90% confluence) using MTT assay (Zhao et al., 2005). The solution of 0.1 nM E2 was used as a positive control and 0.1% dimethyl sulfoxide as a negative control. The absorbance was measured at 570 nm with background subtractions at 630 nm using a microplate reader (Dynex/MRX microplate reader, Dynex Technologies, Inc., VA, USA). The relative proliferative effect was calculated with the following equation:
Relative proliferative effect (%) = Absorbance of sample/Absorbance of E2 × 100
Cytotoxicity study
Cytotoxicity of herbal extracts was investigated in mouse fibroblast cells using MTT assay (Talebi et al., 2006). The cells were routinely maintained in DMEM, supplemented with 10% FBS, 1% Penicillin/Streptomycin, and were incubated in a humidified atmosphere of 5% CO2 at 37°C. The confluent cells were washed with sterile isotonic phosphate buffer saline solution and were trypsinized with 0.25% trypsin/EDTA solution. The cells were transferred in each well of 96-well plates. Doxorubicin HCl was used as a positive control. The cytotoxic effects were expressed as
a percentage of cell survival as follows:
Cell viability (%) = Absorbance of the tested sample/Absorbance of the medium only × 100
Protective effect against H2O2-induced oxidative stress
The protective effects of selected phytoestrogenic extracts on the H2O2-induced oxidative stress were evaluated in mouse fibroblast cells using MTT assay (Jitsanong et al., 2011). The cells were seeded in a 96-well culture plate at density of 1 × 104 cells/well. The cells were allowed to attach to the plate for 24 h and were then treated with various concentrations of those extracts for 2 h. After that, H2O2 (1000 µM final concentration) was added to the plate and incubated for an additional 24 h. The cell viability was measured using MTT assay. Cellular morphology was also observed under light microscopy after staining the cells with crystal violet.
Statistical analysis
Data of experimental investigation were analyzed by one-way analysis of variance. In all cases, a minimal level of significance was set at p<0.05 using SPSS software version 15 for Windows (SPSS Inc., Chicago, USA).
RESULTS
Evaluation of Estrogen like activities
A total of 13 herbal medicines belonging to 7 families were selected for this search for extracts with potential estrogenic activity. Their traditional uses are listed in Table 1. These herbal medicines were extracted with 95 %v/v ethanol with percentages of the obtained yields ranging from 3.26 to 15.40 %w/w (Table 1). The estrogen-like activity of 13 herbal extracts was examined in MCF-7 cells and the results are shown in Figure 1. Ten extracts exhibited a growth-promoting effect in MCF-7 cells. The extract of P. candollei var mirifica gave the highest level in growth promoting activity. It significantly stimulated cell proliferation at concentrations of 0.1-50 µ/ml (p<0.05) whereas higher concentration (100 µg/ml) suppressed the growth of such cells. The maximal proliferative effect of this extract was achieved at 50 µg/ml which is higher than the effect displayed by 0.1 nM E2. The extract of P. granatum pericarp exhibited the lowest estrogen-like effect. Overall, the estrogenic activity of all test extracts could be classified into 3 groups:
Table 1. List of selected herbal medicines used for screening estrogen-like activity.
| Scientific name | Family | Used part | Traditional use | Yield (%) |
| P. candollei var mirifica (a) | Leguminosae | Tuberous root | Treatment of post-menopausal symptoms | 11.87 |
| L. usitatissimum (a) | Linaceae | Seed | Treatment of post-menopausal symptoms | 15.78 |
| G. max (a) | Leguminosae | Seed | Treatment of post-menopausal symptoms | 11.20 |
| C. aeruginosa(b) | Zingiberaceae | Rhizome | Restoration of normal menstrual cycleAlleviation of menstrual symptomsTreatment of post-menopausal symptoms | 14.73 |
| C. quadrangularis (a) | Vitaceae | Stem | Improvement of bone fracture healing | 9.02 |
| T. godefroyanum (b) | Leguminosae | Root | Restoration of normal menstrual cycle | 10.40 |
| C. comosa (a) | Zingiberaceae | Rhizome | Alleviation of menstrual symptomsTreatment of post-menopausal symptoms | 15.35 |
| T.a foenum-graecum (a) | Leguminosae | Seed | Increase inadequate breast milk supply | 3.26 |
| P. granatuma (a) | Punicaceae | Fruit | Revitalization of mature skin | 15.40 |
| B. superba (b) | Leguminosae | Root | Rejuvenation | 3.70 |
| T. cruciatum (b) | Vitaceae | Whole part | Treatment of hot flash in post-menopausal women | 10.70 |
| C. argentatum (b) | Annonaceae | Root | Increase inadequate breast milk supply | 3.26 |
| E. hirta (b) | Euphorbiaceae | Whole part | Increase inadequate breast milk supply | 3.30 |
(a) Herbal medicine has been previously reported to contain phytoestrogens.
(b) Herbal medicine has not been reported to have estrogenic activity.
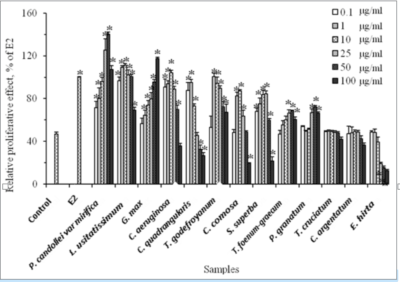
Figure 1. Effects of types and concentrations of 13 herbal extracts on the proliferation of MCF-7 cells (*p<0.05 versus the negative control (C)). The results are expressed as the relative proliferative effect of E2 (0.1 nM, 100%) (n=3).
Estrogen like activities
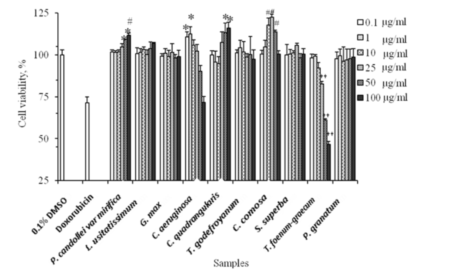
Figure 2. Effects of types and concentrations of 10 herbal extracts on viability of mouse fibroblast cells. (*p<0.05, #p<0.01 and ††p<0.001 versus untreated cells) (n=3).
strong estrogenicity (P. candollei var mirifica, L. usitatissimum, G. max), (2) moderate estrogenicity (C. aeruginosa, C. quadrangularis, T. godefroyanum, C. curcuma, B. superba), and (3) weak estrogenicity (T. foenum-graecum, P. granatum). The remaining 3 extracts namely T. cruciatum, C. argentatum and E. hirta were excluded from this study because they did not exert either proliferative or anti-proliferative effect in the cells. To evaluate the possible mechanism of the action of 10 extracts on the growth of MCF-7 cells, co-treatment of the cells with each herbal extract plus estrogen receptor antagonist (10 nM ICI 182780) was examined. In the absence of this inhibitor, all extracts significantly stimulated the growth of MCF-7 cells in a dose-dependent manner at all test concentrations (data not shown). Neither the cell treated with E2 nor herbal extracts in combination with ICI 182780 had any effect on the growth of the cells. These co-treatments led to a decrease in the value of the relative proliferative effect by almost 50% of E2 (p<0.05). The values obtained were not significantly different compared to those of untreated cells. These results indicated that the phytoestogenic substances in the extracts exerted their estrogenic activities through estrogen receptor pathways.
Cytotoxicity study of herbal extracts
The cytotoxicity of 10 herbal extracts was investigated in mouse fibroblast cells using MTT assay. Figure 2 illustrates the cytotoxicity of those extracts in term of cell viability to untreated cells. Cell viability of doxirubin HCl (20 µg/ml, positive control) was about 70% for all experiments. Among 10 extracts tested, 8 extracts did not show detectable cytotoxicity over the tested concentra-tions (1, 10 and 100 µg/ml). However, cytotoxic effects were observed when the cells were treated with high concentrations of C. aeruginosa extract (50 and 100 µg/ml) and T. foenum-graecum (25, 50 and 100 µg/ml). A high concentration of P. candollei var mirifica (25 µg/ml or higher) could significantly stimulate the growth of the cells as compared to untreated cells with a cell viability of 104.84±1.84% (p<0.05), 109.34±0.38% (p<0.05) and 111.80±1.34% (p<0.01) for 25, 50 and 100 µg/ml. C. quadrangularis extract promoted the growth of cells to 113.33±5.75% and 116.01±3.62% of control at 50 and 100 µg/ml, respectively. C. comosa extract at concentrations between 10-50 µg/ml stimulated cell growths while no effect was observed at 100 µg/ml. At a low concentration (0.1 and 1 µg/ml), C. aeruginosa extract promoted cell growth. Four extracts of L. usitatissimum seeds, G. max seeds, T. godefroyanum roots and P. granatum pericarp showed no effect on the growth or death of the cells (p>0.05). Therefore, 9 extracts represented as promising candidates for cosmetic use. T. foenum-graecum did not.
Protective effect against H2O2-induced oxidative stress
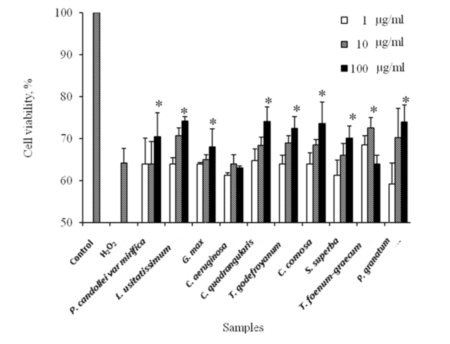
Figure 3. Protective effects of 10 herbal extracts on the H2O2-induced oxidative stress in mouse fibroblast cells. (C, 0.1% DMSO; H2O2, 1000 µM H2O2. p<0.05 versus the negative control (C)) (n=3).
The protective effect of 10 herbal extracts against oxidative stress induced by H2O2 was investigated in mouse fibroblast cells. Results showed that cell survival was slightly increased in a dose dependent manner (Figure 3). No significant increase in cell viability was observed for cells pre-treated with 1 µg/ml of all herbal extracts (p>0.05). However, the viabilities of cells pre-treated with 10 µg/ml in L. usitatissimum and T. foenum-graecum increased to 70.73±1.82 and 72.56±2.51% of control. The highest level for cell viabilities was observed when cells were pre-treated with 100 µg/ml herbal extracts. These results suggested that 10 herbal extracts could protect against cell death under oxidative stress.
DISCUSSION
The study was designed to search for herbal extracts with potential estrogenicity to be used as biologically active principles in cosmetic preparations. Of the 13 herbal extracts, 10 displayed a biphasic response in the growth of MCF-7 cells. The growth-promoting effect of these plants was blocked by co-treatment with ICI 182780. These screening results suggested that the 10 extracts had estrogen properties and could mediate their activities through the activation of estrogen receptors in these cells. P. candollei var mirifica, L. usitatissimum and G. max showed the strongest estrogenic activity, followed by C. aeruginosa, C. quadrangularis, T. godefroyanum, C. comosa, B. superba, T. foenum-graecum and P. granatum. The differences in estrogenic activities of these herbs could be caused by their phytoestrogenic contents and chemical structures. Among the above-mentioned extracts, C. aeruginosa and T. godefroyanum were suggested as new herbal medicines having estrogenicity. This should be further confirmed for their estrogenicities with other assays because the results obtained from E-screen assay could exert through other pathways than those involving transcriptional activation of estrogen responsive genes. Three extracts, T. cruciatum, C. argentatum and E. hirta, showed neither proliferative nor antiproliferative effects on such cells, indicating they did not contain phytoestrogens. It was noted that the growth of MCF-7 cells could either be stimulated or inhibited depending upon the tested concentration of the phytoestrogenic extracts. These findings were in agreement with previous reports. On the contrary, they inhibited the growth of both estrogen positive and negative cell lines at high concentrations (Bou et al., 2003). P. candollei var mirifica mediated its action at a very low concentration compared to other extracts, indicating it was the strongest estrogenic herb. The tuberous root of this herb has been traditionally consumed for rejuvenation and treatment of post-menopausal symptoms in Thailand. The data were in agreement with those reported by other researchers (Cherdshewasart et al., 2004). The high performance liquid chromatography (HPLC) analysis confirmed P. candollei var mirifica carried isoflavonoids as major components (puerarin, daidzin, genistin, daidzein, genistein, coumestrol) (data not shown). Moreover, other researchers reported that its chromene (that is miroestrol, dexoymiroestrol) exhibited stronger binding affinity for estrogen receptor α than the isoflavonoids (Sugiyama et al., 2009). Thus, the high content of these isoflavonoids could contribute to its high estrogenic effect, explaining the higher estrogenicity of P. candollei var mirifica than other plants. The herbal medicines being used to restore normal menstrual cycles and alleviate menstrual symptoms in pre-menopausal women, such as L. usitatissimum, G. max, C. aeruginosa, C. comosa and T. godefroyanum, showed the ability to promote MCF-7 cell proliferation. L. usitatissimum and G. max extracts showed better estrogenicity than other herbs in this group. In nature, seeds of L. usitatissimum contain high content of lignans (that is isolaricinresinol, pinoresinol, secoisolariciresinol, matairesinol) which are recognized as potent phytoestrogens (Attoumbré et al., 2011). These compounds could bind to estrogen receptors in MCF-7 cells, resulting in stimulated cell growth. The exact substances in C. comosa were not identified in this work. However, the presence of diarylheptanoids in rhizomes of C. comosa extract could be responsible for its estrogenicity (Suksamrarn et al., 2008). The extract obtained from pericarp of P. granatum displayed very weak estrogenic activity. This activity could be attributed to the action of their major phytoestrogenic compounds (that is luteolin, quercetin, kaempferol) (van Elswijk et al., 2004). Moreover, ellagic acid, one of the components of this plant, has been shown to exhibit both estrogenic and anti-estrogenic effects in MCF-7 cells (Strati et al., 2009). With respect to herbal medicines traditionally used as a galactogogue to increase inadequate breast milk supply, only T. foenum-graecum promoted the growth of MCF-7 cells in a dose-dependent manner, while there was no observed effect for C. argentatum and E. hirta. This finding was in agreement with data reported by other authors that chloroform extract of T. foenum-graecum promoted the growth of MCF-7 cells (Sreeja et al., 2010).
It induced the transcription of estrogen-responsive gene pS2 in MCF-7 cells (gene marker for assessing estrogenicity). Several bioactive compounds, such as formononetin, quercetin, and apigenin, and their derivatives could exert their estrogenic effects in such cell lines (Rayyan et al., 2010). Interestingly, the ethanolic extract of B. superba and its main isoflavones (that is prunetin, medicarpin, formononetin, 7-hydroxy-6-4´-dimethoxyisoflavone, 7,4´-dimethoxyisoflavone, and hexacosanoic acid 2,3-dihydroxy-propyl ester) showed weak estrogenic property in recombinant yeast screening assay (Cherdshewasart et al., 2010). It has been reported previously that B. superba could not promote the growth of MCF-7 cells (Cherdshewasart et al., 2004). This discrepancy could be caused by the difference in culture media used for testing the growth-promoting effect. In that study’s preparation, DMEM with phenol red and fetal bovine serum containing estrogens were used, different from this study in which phenol red-free DMEM and charcoal-coated dextran stripped fetal bovine serum were used. The phenol red can act as an agonist for estrogen receptor in MCF-7 cells. To evaluate the estrogenic effect of herbal medicines, the estrogen or phenol red should be discarded from the culture medium. Thus, it should be possible to mention that the B. superba displayed estrogenic property because it exerts biphasic response in such a cell line and its active ingredient was previously identified as phytoestrogens (Cherdshewasart et al., 2010). The safety of these plant extracts is an important task that needs to be first evaluated for possible cosmetic benefit. The preliminary evaluation for their cytotoxic effects was carried out in mouse fibroblasts. It is well documented that plant extracts possess low cytotoxicity if the relative viability of the cells after exposure to 100 µg/ml extract is higher than 80% of negative control (Wang et al., 2006). Of the 10 tested extracts, C. aeruginosa and T. foenum-graecum were toxic on mouse fibroblasts by reduced cell viability lower than 80% of negative control at 100 µg/ml. The use of H2O2 has been reported as an alternative technique for studying the protective effect of herbal extracts against oxidative stress. Mammalian cells generally exposed to H2O2 exhibit apoptotic feature, such as shrinkage in their morphologies and nuclear fragmentations. Interestingly, pre-treatment of those 10 extracts to the fibroblast cells prior to the addition of H2O2 reduced the apoptotic cells as well as increased the percentage in cell survival. These findings indicated the potentially protective effect of herbal extracts against oxidative stress-induced cell death. The mechanism of these extracts should partly associate with their radical scavenging activities. Previous reports confirmed these findings that phytoestrogens or phytoestrogenic extracts protected cells from oxidative-induced cell death (Jitsanong et al., 2011).
Conclusion
This work demonstrated that 10 extracts exhibited estrogen-like effect in MCF-7 cells. These were P. candollei var mirifica, L. usitatissimum, G. max, C. aeruginosa, C. quadrangularis, T. godefroyanum, C. comosa, B. superba, T. foenum-graecum and P. granatum. All except T. foenum-graecum were promising candidates as active principles for anti-skin-ageing in post-menopausal women. These herbal extracts exhibited either growth-promoting or -inhibiting properties. It was possible that the difference in estrogenic activity of all extracts was caused by the presence of differently estrogenic compounds.
ACKNOWLEDGEMENTS
This study was partially supported by a grant from the Thai Graduate Institute of Science and Technology (TGIST, TG-55-24-49-070D to B. Yingngam) and by the Ubon Ratchathani University, Thailand. Special thanks are also extended to Bob Tremayne for English editing of the manuscript.
Estrogen like activities
REFERENCES
Attoumbré J, Laoualy ABM, Bienaimé C, Dubois F, Baltora-Rosset S (2011). Investigation of lignin accumulation in developing Linum usitatissimum seeds by immunolocalization and HPLC. Phytochem. Lett., 4: 194-198.
Boonchird C, Mahapanichkul T, Cherdshewasart W (2010). Differential binding with ERβ and ERβ of the phytoestrogen-rich plant Pueraria mirifica. Braz. J. Med. Biol. Res., 43: 195-200. Bou SM, Wiese TE, Nehls S, Burow ME, Elliott S, Carter-Wientjes CH, Shih BY, McLachlan JA, Cleveland TE (2003). Evaluation of the estrogenic effects of legume extracts containing phytoestrogens. J. Agric. Food Chem., 51: 2193-2199. Cherdshewasart W, Cheewasopit W, Picha P (2004). The differential anti-proliferation effect of white (Pueraria mirifica), red (Butea superba), and black (Mucuna collettii) Kwao Krua plants on the growth of MCF-7 cells. J. Ethnopharmacol. 93: 255-260. Cherdshewasart W, Mahapanichkul T, Boonchird C (2010). Estrogenic and anti-estrogenic activities of the Thai traditional herb, Butea superba Roxb. Biosci. Biotechnol. Biochem., 74: 2176-2186. Jitsanong T, Khanobdee K, Piyachaturawat P, Wongprasert K (2011). Diarylheptanoid 7-(3,4 dihydroxyphenyl)-5-hydroxy-1-phenyl-1(E)-1-heptene from Curcuma comosa Roxb. Protects retinal pigment epithelial cells against oxidative stress-induced cell death. Toxicol. In Vitro, 25: 167-176. Lee SH, Jin N, Paik DJ, Kim DY, Chung IIIM, Park Y (2011). Consumption of legumes improves certain bone markers in ovariectomized rats. Nutr. Res., 31: 397-403.
Miyazaki K, Hanamizu T, Sone T, Chiba K, Kinoshita T, Yoshikawa S (2004). Topical application of Bifidobacterium-fermented soy milk extract containing genistein and daidzein improves rheological and physiological properties of skin. J. Cosmet. Sci., 55: 473-479. Rayyan S, Fossen T, Andersen ØM (2010). Flavone C-glycosides from seeds of fenugreek, Trigonella foenum graecum L. J. Agric. Food Chem., 58: 7211-7217. Sreeja S, Anju VS, Sreeja S (2010). In vitro estrogenic activities of fenugreek Trigonella foenum graecum seeds. Indian J. Med. Res., 131: 814-819. Strati A, Papoutsi Z, Lianidou E, Moutsatsou P (2009). Effect of ellagic acid on the expression of human telomerase reverse transcriptase (hTERT) α+β+ transcript in estrogen receptor-positive MCF-7 breast cancer cells. Clin. Biochem., 42: 1358-1362. Südel KM, Venzke K, Mielke H, Breitenbach U, Mundt C, Jaspers S, Koop U, Sauermann K, Knussman-Hartig E, Moll I, Gercken G, Young AR, Stäb F, Wench H, Gallinat S (2005). Novel aspects of intrinsic and extrinsic aging of human skin: beneficial effects of soy extract. Photochem. Photobiol., 81: 581-587. Sugiyama H, Kumamoto T, Suganami A, Nakanishi W, Sowa Y, Takiguchi M, Ishikawa T, Tamura Y (2009). Insight into estrogenicity of phytoestrogens using in silico simulation. Biochem. Biophys. Res. Commun., 379: 139-144. Suksamrarn A, Ponglikitmongkol M, Wongkrajang K, Chindaduang A, Kittidanairak S, Jankam A, Yingyongnarongkul B, Kittipanumat N, Chokchaisiri R, Khetkam P, Piyachaturawat P (2008). Diarylheptanoids, new phytoestrogens from rhizomes of Curcuma comosa: isolation, chemical modification and estrogenic activity evaluation. Bioorg. Med. Chem., 16: 6891-6902. Talebi S, Afshari JT, Rakhshandeh H, Seifi B, Boskabadi MH (2006). In vitro antiproliferative effect of fresh red garlic on human transitional cell carcinoma (TCC-5637 cell line). Inter. J. Agric. Biol., 8: 609-614. Tomaszewski J, Adamiak A, Skorupski P, Rzeski W, Rechberger T (2003). Effect of 17 beta-estradiol and phytoestrogen daidzein on the proliferation of pubocervical fascia and skin fibroblasts derived from women suffering from stress urinary incontinence. Ginekol. Pol., 74: 1410-1414. van Elswijk DA, Schobel UP, Lansky EP, Irth H, van der Greef J (2004). Rapid dereplication of estrogenic compounds in pomegranate (Punica granatum) using on-line biochemical detection coupled to mass spectrometry. Phytochem., 65: 233-241. Wang KH, Lin RD, Hsu FL, Huang YH, Change HC, Huang CY, Lee MH, (2006). Cosmetic applications of selected traditional Chinese herbal medicines. J. Ethopharmacol., 106: 353-359. Zhao QW, Li B, Weber N, Lou YJ, Proksch P (2005). Estrogen-like effects of ethanol extracts from several Chinese legumes on MCF-7 cell. Eur. Food Res. Technol., 221: 828-833.
Bancha Yingngam1 , Nuttapun Supaka2 and Wandee Rungseevijitprapa1
1 Department of Pharmaceutical Chemistry and Technology, Faculty of Pharmaceutical Sciences, Ubon Ratchathani University 34190, Thailand.
2 National Nanotechnology Center, National Science and Technology Development Agency, Thailand Science Park, Pathumthani 12120, Thailand.
Accepted 26 October, 2011
keyword: Estrogen like activities , Estrogen like activities , Puerariamirifica, Estrogen like activities, Pueraria, Estrogen like activities, Estrogen like activities , Estrogen like activities , Estrogen like activities







