Antitumor activity of spinasterol isolated from Pueraria roots
Abstract
We purified phytoestrogens from Pueraria root (Pueraria Mirifica from Thailand and Pueraria lobata from Korea), which is used as a rejuvenating folk medicine in Thailand and China. Dried, powdered plant material was extracted with 100% ethanol and further separated by concentration, filtration, and thin layer silica gel chromatography. Using the fractions obtained during separation, we first investigated their cytotoxicity in several cancer cell lines from various tissues. The ethanol-extracted components (PE1, PE4) had significant antiproliferative effects on breast cancer cell lines, including MCF-7, ZR-75-1, MDA-MB-231, SK-BR-3, and Hs578T. Second, we compared these results with the cytotoxic effects of known flavonoids, sterols, and coumarins from Pueraria root. The known compounds were not as effective, and occurred in a different polarity region on HPLC. Third, further separation resulted in the isolation of eight different components (Sub PE-A to -H). One of these, PE-D, affected the growth of some breast cancer cell lines (MCF-7, MDA- MB-231) in a dose- and time-dependent manner, as well as the growth of ovarian (2774) and cervical cancer cells (HeLa). Finally, a transfection assay showed that this component had an estrogenic effect similar to 17β – estradiol, which activates both estrogen receptor α (ERα) and ERβ. The NMR analysis determined that spinasterol (stigmasta-7, 22-dien-3beta-ol) is an active cytotoxic component of Pueraria root.
Keywords: breast neoplasms; estrogen replacement; phytoestrogens; pueraria; receptors, estrogen; spinasterol; therapy
Introduction
Estrogen exerts a wide variety of effects on growth, development, differentiation, and reproduction (for review, see Nilsson et al., 2001). Estrogen mediates these activities via binding to a specific nuclear receptor protein, the estrogen receptor (ER), which is encoded by two genes (ERα and ERβ) that function as transcription factors to regulate the expression of target genes (Osborne et al., 2001). On ligand binding, ER undergoes conformational changes and dissociates from the inactive R-hsp90 complex. The activated ER enters the nucleus as a homodimer or heterodimer, then binds to a specific DNA sequence, the estrogen response element (ERE), and stimulates estrogen-target gene expression. The two ERs appear to have unique tissue distributions and their own sets of specific functions. Knowledge of these functions might aid in the development of receptor-specific selective estrogen receptor modulators (SERMs) (Kuiper and Gustafsson, 1997; Barkhem et al., 1998; Nilsson et al., 1998).
Estrogen replacement therapy (ERT) is used to treat symptoms of menopause, such as hot flashes and osteoporosis, and to reduce the incidence of cardiovascular disease associated with menopause (This et al., 2001). However, current ERT appears to be associated with increased risks of developing breast and ovarian cancer in healthy women (Schairer et al., 2000; Lacey et al., 2002). To overcome the shortcomings of ERT, phytoestrogens derived from plants have emerged as an alternative to ERT to alleviate the symptoms of menopause, although their safety needs to be evaluated further (Tham et al., 1997).
Pueraria root prepared from Pueraria mirifica or Pueraria lobata is one of the most important crude drugs in traditional oriental medicine. For example, the Thai vine, Pueraria mirifica, is used as a rejuvenator and aphrodisiac (Bradbury and White, 1954; Cain, 1960), and other Pueraria species are reported to have pharmacological activity (Harada and Ueno, 1975; Qicheng, 1980; Lai and Tang, 1989; Keung and Vallee, 1998; Miyazawa et al., 2001). Pueraria root is a potent source of phytoestrogen with estrogen-like biological activity. Three main classes of phytoestrogens occur in either plants or their seeds: isoflavones, coumestans, and lignans. A single plant often contains more than one class of phytoestrogen. It has been suggested that the major estrogenic components of Pueraria root are puerarin, daidzin, genistin, daidzein, and genistein (Ohshima et al., 1988). No powerful phytoestrogens other than these isoflavones and coumestans have been isolated. In addition, their activities in the treatment of gynecological cancers are poorly defined.
In this study, we extracted phytoestrogens from two kinds of Pueraria root: Pueraria mirifica from Thailand and Pueraria lobata from Korea. We used breast, ovarian, and cervical cancer cell lines to analyze their chemopreventive activity in gynecological cancers in vitro, and determined their effect on ERα and ERβ. We found that the profiles of the phytoestrogens extracted from the two Pueraria roots were similar, and demonstrated that Pueraria extract has antiproliferative effects on certain gynecological cancer cell lines, and acts on both ERs. The active cytotoxic component in the final fraction appears to be spinasterol, an isomer of stigmasterol, which is one of the known phytoestrogens in Pueraria extract. Although a more detailed approach is required to determine how spinasterol regulates the growth of cancer cells, our results may aid the development of natural SERMs for menopausal women who need ERT, without increasing their risk of developing gynecological cancers.
Materials and Methods
Preparation of Pueraria extracts
Pueraria roots were extracted by inverted shaking in 100% ethanol for 3 days at room temperature. A clear extract was recovered by centrifugation at 5,000 rpm for 20 min and concentrated in a rotary evaporator at 45C (PE1; Pueraria extract 1). After PE1 was filtered, the filtrate was concentrated and filtered again to produce PE4. To separate it further, PE4 was subjected to preparative thin-layer chromatography (TLC) on Silica Gel 60 F254 (Merck, Darmstadt, Germany) with hexane:ethyl acetate (4:1). Eight sub-fractions were recovered, of which Sub PE-D was further separated by preparative TLC (hexane:ethyl acetate = 5:1) to produce Sub PE-D1, -D2 and -D3. To identify the structures in the final three fractions, the fractions were subjected to NMR (proton and carbon) and LC-MASS. The 1H NMR and 13 C NMR spectra were recorded on a JEOL JNM EX-400 using CDCl3 as the solvent. All the chemical shifts (δ) are quoted in ppm downfield from TMS and the coupling constants (J) are given in Hz. Mass spectra were measured on a Shimazu GCMS-PO 1,000 mass spectrometer (EI 70 eV). The dried material derived from each fraction was dissolved in DMSO (Sigma, St. Louis, MO), 100% ethanol, or CH2Cl2, depending on the experimental conditions, and stored at -20C. To treat cells, the dissolved material was diluted with phenol red- free DMEM (Gibco BRL, Gaithersburg, MD) to give the final concentrations indicated in the text.
Cell lines and cell culture
The cells used in our experiments were breast (MDA- MB-231, MCF-7, ZR-75-1, Hs578T, SK-BR-3), cervical (HeLa, CaSki), ovarian (2774, OVCAR-3, PA-1, SK- OV-3), colon (HT-29, SW480, HCT116, DLD-1), and liver (PLC/PRF-5, SK-Hep-1) cancer cell lines. These cells were routinely maintained in DMEM, supplemented with 10% FBS) (HyClone Laboratories, Logan, UT), previously inactivated at 56C for 20 min. For transfection and transcription assays, COS-1 cells were used and cultured in phenol red-free DMEM, supplemented with heat-inactivated, charcoal-treated 10% FBS.
Cell viability
Cell monolayers were cultured in a humidified atmosphere of 95% air with 5% CO2. Cells were seeded at a density of 7 × 10 4 cells/well 24 h before drug treatment in six-well plates, and were 60 to 70% confluent at the time of treatment. Different concentrations of the Pueraria extracts or commercially available phytoestrogens (puerarin, stigmasterol, campesterol, coumestrol, genistein, daidzein, daidzin, genistin, β -sitosterol) (Sigma) were added to the medium. As a control, a stock solution of 100 mM 17β- estradiol (Sigma) dissolved in DMSO, 100% ethanol, or CH2Cl2 was used. After treatment, living cells were recovered daily by suspension in trypsin-EDTA and counted using a hemocytometer followed by trypan blue staining (0.4%). Each assay was performed four times in triplicate. All the data presented in the text represent the means of three independent assays.
Transcription assay for ERα and ERβ
Cells were transfected using a liposome-based method with Lipofectamine (Gibco-BRL), as previously reported (Um et al., 2000; Kang et al., 2004). Briefly, COS-1 cells, maintained in phenol red-free DMEM supplemented with charcoal-treated 10% FBS, were plated in 60-mm dishes at a density of 1 × 10 6 cells/ dish 5 h before transfection. After overnight transfection with the indicated reporter plasmids (ERα or ERβ expression vector and Vit-tk-CAT reporter plasmid derived from vitellogenin promoter containing a canonical ER response element; generously donated by Dr. Pierre Chambon, Strasbourg, France) together with SV40-driven internal control plasmid, the cells were washed, fed with complete medium if needed, treated with 17β-estradiol or Pueraria extract, Antitumor activity of spinaterol 113 and further incubated for 24 h. Cell extracts were then prepared and the β-galactosidase activity was determined to normalize transfection. The CAT concentration in 30-70 µl of the clear lysate was tested using CAT ELISA according to the manufacturer’s instructions (Roche Molecular Biochemicals, Mannheim, Germany). The data given in the text are the means of at least three independent transfections. The relative amount of CAT was calculated by dividing the amount of CAT enzyme in the treated cells by that in untreated cells.
Statistical analysis
Data are the means of at least three different experiments. The significance of the differences between groups was determined by one-way ANOVA, with the software SPSS (Version 9.0.1, SPSS, Chicago, IL) and Student’s t-test. A P-value<0.05 was considered statistically significant.
Results and Discussion
After we found that the starting extract of Pueraria powder (PE1) showed strong antiproliferative activity in specific gynecological cancer cells (data not shown), we further fractionated PE1 to produce PE2, PE3, and PE4 (Figure 1). The dose-dependent cytotoxic effect of the extracts was measured by counting viable cells directly. As shown in Figures 2A and 2B, the growth of both MCF-7 and MDA-MB-231 breast cancer cells was specifically reduced by PE4. The control, 17β- estradiol (E2), was hyperproliferative at 1 µM and apoptotic to MCF-7 at higher concentrations, as previously reported (Reddel and Sutherland, 1987; Kim et al., 2000). The strong antiproliferative activity of PE4 prompted us to investigate its effect on other breast cancer cells: ZR-75-1, HS578T, SK-BR-3, and T47D. All the cells except ZR-75-1 were sensitive to PE4 in a dose-dependent manner (Figures 2C-2F). The cytotoxic effect of PE4 appears to be ERα-independent, since MCF-7 and ZR-75-1 are ERα-positive, while the other cell lines are ERα-negative or ERβ- positive.

PE indicates Pueraria extract.
To get a clue as to the components involved in the response to PE4, we tested the cytotoxicity of known components of Pueraria root (puerarin, stigasterol, campesterol, coumestrol, genistein, daidzein, daidzin, genistin, β-sitosterol) on MCF-7 and MDA-MB-231 breast cancer cells. None of these were cytotoxic at up to 10 µM (data not shown). To further confirm that the active cytotoxic components of PE4 are not known components of Pueraria root, they were characterized physically and chemically (data not shown). Reverse-phase TLC and HPLC using a µBondapak C18 column (Shimadzu, Kyoto, Japan) indicated that the active components of PE4 are extremely nonpolar, whereas the nine known compounds are relatively polar, except stigmasterol, campesterol, and β-sitosterol. The latter three compounds were not detected under UV. Like the components in PE4, these three stained with anisaldehyde-sulfuric acid and molybdophosphoric acid, which were used to determine the presence of a phenol ring and steroid, respectively. These results suggested that the active cytotoxic component(s) of PE4 is structurally similar to, but not the same as, stigmasterol, campesterol, and β-sitosterol.



Table 1. Summarized cytotoxic effects of Pueraria extracts on various cancer cell lines.

From the results shown in Figure 2, we realized that PE2 and PE3 had little effect, whereas PE1 and the final filtrate, PE4, did have an effect on most of the breast cancer cells tested. Moreover, the physicochemical characterization of PE4 further separatedthe components in PE4; PE4 was subjected to preparative TLC on Silica Gel 60 F254 in hexane: ethylacetate (4:1). To determine the effect of the sub-fractions of PE4 on gynecological cancer cells, including two breast cancer cells, cell lines were exposed to each sub-fraction at a concentration of 10 µM, which corresponds to 4.17 mg/ml, based on the average molecular weight (412.55) of the nine known components of Pueraria root (Figure 3). PE4 was used as a positive control. When compared with ethanol treatment (shown as C), sub-fraction D of PE4 (Sub-D) reduced the numbers of the two breast cancer cells greatly. Of the two ovarian cancer cell lines, 2774 was sensitive to Sub PE-D, whereas SK-OV-3 was not. Similarly, of the two cervical cancer cell lines, HeLa was responsive to Sub-D, whereas CaSki was not.
To measure the IC50, the concentration inhibiting growth by 50%, cultured MDA-MB-231 cells were treated with each derivative at six different concentrations (0, 0.5, 1, 2, 5, and 10 µM). As shown in Figure 4A, the IC50 of Sub PE-D was 6 µM. In the presence of 10 µM, the growth of MDA-MB-231 cells was completely inhibited (Figure 4B). Again, the growth of 2774 and HeLa cells was greatly inhibited by Sub PE-D in a dose-dependent manner (Figure 4C). The cytotoxic effects of the extracts of Pueraria root are summarized in Table 1. Interestingly, the Pueraria extracts had no effect on the colon or liver cancer cell lines used, or on normal human fibroblasts and Chang liver (epithelium-originated) cells. These results indicate that the Sub PE-D fraction contains the effective component(s) of Pueraria root, which is cytotoxic in the selected gynecological cancer cell lines used in this study, but not in normal cell lines. However, we did not determine which component(s) of Sub PE-D is responsible for the cytotoxicity. Additional work is required, including the further separation and structural determination of the isolated component(s).
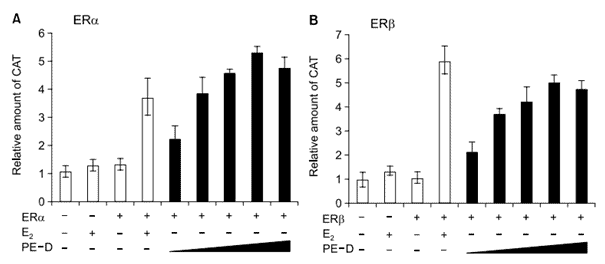
To determine whether the component(s) of Sub PE-D acts as phytoestrogen, we performed transcription assays in which COS-1 cells were transiently transfected with Vit-tk-CAT reporter plasmid, and ERα or ERβ expression vector. As a positive control, 17β estradiol (E2) was used. As expected, 1 mM E2 activated the transcriptional activities of both ERα and ERβ. When increasing concentrations of Sub PE-D were used, it was as active as E2 for ERα, and a little less active than E2 for ERβ (Figure 5). In another assay, no E2 antagonist effect of Sub PE-D was observed (data not shown). These results suggest that the Sub PE-D fraction of Pueraria root contains a phytoestrogen that activates both ERα and ERβ as an E2 agonist. However, it is not clear which component(s) of Sub PE-D acts as a phytoestrogen.
To determine which component(s) of Sub PE-D is responsible for its cytotoxicity in breast cancer cells, and constitutes the phytoestrogen that activates ER, Sub PE-D was further subjected to preparative TLC in hexane:ethylacetate (5:1). From this sub-fractionation, we obtained Sub PE-D1, -D2, and -D3. As shown in Figure 2, ZR-75-1 cells were not sensitive, whereas MCF-7, MDA-MB-231, and HeLa cells were sensitive to Sub PE-D. Of the sub-fractions, PE-D2 inhibited the growth of cancer cells as much as Sub PE-D (Figure 6), indicating that PE-D2 contains the active cytotoxic component of Sub PE-D.
1H NMR and 13C NMR spectra of Sub PE-D2 were obtained. : 1 H NMR (CDCl3, 400 MHz) : δ 0.68~0.79 (3H, t, J=6.8 Hz), 0.81-0.86 (10H, m), 0.91-0.98 (5H, m), 1.01-1.03 (6H, m), 1.07-1.25 (6H, m), 1.28-1.41 (7H, m), 1.48-1.68 (10H, m), 1.83-1.86 (3H, m), 1.95-2.02 (3H, m), 2.23-2.29 (2H, m), 3.52-3.53 (1H. m), 5.36 (1H. s); 13C NMR (CDCl3) : δ 11.86, 11.99, 18.79, 19.04, 19.40, 19.83, 21.08, 23.06, 24.31, 26.05, 28.25, 29.14, 31.65, 31.90, 33.93, 36.16, 36.50, 37.25, 39.77, 42.29, 42.32, 45.82, 50.12, 56.25, 56.77, 71.79, 121.72, 140.75. Mass spectroscopy indicated that the molecular weight of Sub PE-D2 was 397 (M-1) and 551 (M+NBA). The NMR and mass data suggest that the active component in Sub PE-D2 is spinasterol (stigmasta-7,22-dien-3beta-ol), an isomer of stigmasterol, which is one of the phytoestrogens present in Pueraria extract. Spinasterol has been reported in Cucurbita moschata (Rodriguez et al., 1996), Conyza blinii (Xu et al., 1998), Gypsophila oldhamiana (Yang et al., 1999), Gordonia ceylanica (Herath et al., 2001), and Acacia cedilloi (Pech et al., 2002). Recently, the anti-tumor activity of spinasterol was demonstrated in vivo in studies that showed that it greatly decreased the incidence of skin tumors without co-carcinogen or co-tumor promoter activities (Villasenor and Domingo, 2000). Little is known of spinasterol as a phyto-estrogen other than that it exists and has anti-tumor activity. In transcription assays with ER, we found that the spinasterol present in Sub PE-D2 acts as an E2 agonist, like Sub PE-D (data not shown), suggesting that it could be a phytoestrogen. Although the active cytotoxic component of Pueraria root was identified as spinasterol, it is still not clear how spinasterol inhibits the growth of specific cancer cell lines such as MCF-7, MDA-MB-231, 2774, and HeLa. More work is required to investigate the mechanisms by which spinasterol influences the cell growth and apoptosis of tumor cells.

We drew the following conclusions from this study. First, Sub PE-D derived from Pueraria extracts showed a strong anti-proliferative effect on some gynecological cancer cell lines, including breast (MCF-7, MDA-MB-231), ovarian (2774), and cervical (HeLa) cell lines, but did not affect normal human fibroblasts or Chang liver cells. Second, Sub PE-D acts as an E2 agonist with preferential activity on ERα over ERβ. However, it is not clear whether ER activation via endogenous ER in sensitive cells is correlated with its cytotoxicity. Third, the active cytotoxic component in the final Sub PE-D2 fraction appears to be spinasterol, an isomer of stigmasterol, which is one of the known phytoestrogens in Pueraria extract. These results suggest that phytoestrogens derived from Pueraria root could be used as a natural alternative to estrogen, offering a lower risk of gynecological cancer when ERT is considered to alleviate the symptoms of menopause. Moreover, the phytoestrogens could be used as a chemopreventive agent in some gynecological cancers.
Acknowledgement
This work was supported by grants from the Plant Diversity Research Center of the 21st Century Frontier Research Program (PF0321001-00) funded by the Ministry of Science and Technology, and from the Ministry of Commerce, Industry and Energy (R-04-021), Republic of Korea. S.J.U. was supported by BK21 project from the Ministry of Education and Human Resources Development.
References
Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson JA, Nilsson S. Differential response of estrogen receptor α and estrogen receptor β to partial estrogen agonist/antagonists. Mol Pharmacol 1998;54;105-12 Bradbury RB, White DE. 1954 Estrogens and related substances in plants. Vitamins Horm 1954;12;207-33 Cain JC. Miroestrol: an oestrogen from the plant Pueraria mirifica. Nature 1960;188;774-7 Harada M, Ueno K. Pharmacological studies on pueraria root. I. Fractional extraction of pueraria root and identification of its pharmacological effects. Chem Pharm Bull (Tokyo) 1975;23;1798-805 Herath HM, Athukoralage PS, Jamie JF. A new oleanane triterpenoid from Gordonia ceylanica. Nat Prod Lett 2001;15; 339-44 Kang JH, Chang SY, Yeom DH, Kim SA, Um SJ, Hong KJ. Weakening of the repressive YY-1 site on the thrombospon-din-1 promoter via c-Jun/YY-1 interaction. Exp Mol Med 2004;36;300-10 Keung WM, Vallee BL. Kudzu root: an ancient Chinese source of modern antidipsotropic agents. Phytochemistry 1998;47;499-506 Kim CJ, Um SJ, Kim TY, Kim EJ, Park TC, Kim SJ, Namkoong SE, Park JS. Regulation of cell growth and HPV genes by exogenous estrogen in cervical cancer cells. Int J Gynecol Cancer 2000;10;157-64 Kuiper GGJM, Gustafsson JA. The novel estrogen receptor-β subtype: potential role in the cell- and promoter-specific actions of estrogens and anti-estrogens. FEBS Lett 1997; 410;87-90 Lacey JV Jr, Mink PJ, Lubin JH, Sherman ME, Troisi R, Hartge P, Schatzkin A, Schairer C. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA 2002;288;334-41 Lai XL, Tang B. Recent advances in the experimental study and clinical application of Pueraria lobata (Willd) Ohwi. Zhongguo Zhong Yao Za Zhi 1989;14;308-11Miyazawa M, Sakano K, Nakamura S, Kosaka H. Anti-mutagenic activity of isoflavone from Pueraria lobata. J Agric Food Chem 2001;49;336-41 Nilsson S, Kuiper GGJM, Gustafsson J-A. ERβ: a novel estrogen receptor offers the potential for new drug development. Trends Endocrinol Metab 1998;9;387-95 Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gust-afsson JA. Mechanisms of estrogen action. Physiol Rev 2001;81;1535-65 Ohshima Y, Okuyama T, Takahashi K, Takizawa T, Shibata S. Isolation and high performance liquid chromatography (HPLC) of isoflavone from the Pueraria Root. Planta Med 1988;54;250-4 Osborne CK, Schiff R, Fuqua SA, Shou J. Estrogen receptor: current understanding of its activation and modulation. Clin Cancer Res 2001;7(12 Suppl);4338s-42s Pech GG, Brito WF, Mena GJ, Quijano L. Constituents of Acacia cedilloi and Acacia gaumeri. Revised structure and complete NMR assignments of resinone. Z Naturforsch [C] 2002;57;773-6Qicheng F. Some current study and research approaches relating to the use of plants in the traditional Chinese medicine. J Ethnopharmacol 1980;2;57-63 Reddel RR, Sutherland RL. Effects of pharmacological concentration and cell cycle kinetics of human breast cancer cell lines in vitro. Cancer Res 1987;47;5323-29 Rodriguez JB, Gros EG, Bertoni MH, Cattaneo P. The sterols of Cucurbita moschata (“calabacita”) seed oil. Lipids 1996; 31;1205-8 Schairer C, Lubin J, Troisi R, Sturgeon S, Brinton L, Hoover R. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA 2000;283;485-91 Tham DM, Gardner CD, Haskell WL. Clinical review 97: Potential health benefits of dietary phytoestrogens: a review of the clinical, epidemiological, and mechanistic evidence. J Clin Endocrinol Metab 1998;83;2223-35 This P, De La Rochefordiere A, Clough K, Fourquet A, Magdelenat H, The Breast Cancer Group of the Institut Curie. Phytoestrogens after breast cancer. Endocr Relat Cancer 2001;8;129-34 Um SJ, Kim EJ, Hwang ES, Kim SJ, Namkoong SE, Park JS. Antiproliferative effects of retinoic acid/interferon in cervical carcinoma cell lines: cooperative growth suppression of IRF-1 and p53. Int J Cancer 2000;85;416-23120 Exp. Mol. Med. Vol. 37(2), 111-120, 2005 Villasenor IM, Domingo AP. Anticarcinogenicity potential of spinasterol isolated from squash flowers. Teratog Carcinog Mutagen 2000;20;99-105 Xu L, Liu J, Min D, Wang S, Zhang Z, Guo D, Zheng K. Chemical constituents of Conyza blinii Levl. Zhongguo Zhong Yao Za Zhi 1998;23;293-5 Yang S, Zhong Y, Luo H, Ding X, Zuo C. Studies on chemical constituents of the roots of Gypsophila oldhamiana Miq. Zhongguo Zhong Yao Za Zhi 1999;24;680-1
EXPERIMENTAL and MOLECULAR MEDICINE, Vol. 37, No. 2, 111-120, April 2005
Gook-Che Jeon1, Myoung-Soon Park2,Do-Young Yoon3, Chul-Ho Shin4,Hong-Sig Sin2 and Soo-Jong Um1,51 Department of Bioscience and Biotechnology/Institute of Bioscience Sejong University Seoul 143-747, Korea 2 Chebigen Inc., 305-B, Chungmugwan Sejong University Seoul 143-747, Korea 3 Laboratory of Cell Biology Korea Research Institute of Bioscience and Biotechnology Daejeon 305-333, Korea 4 Seohae Environment Science Institute Chonbuk National University Total Research Complex Jeonbuk 865-854, Korea 5 Corresponding author: Tel, 82-2-3408-3641; Fax, 82-2-3408-3334; E-mail, umsj@sejong.ac.kr
Accepted 23 March 2005
Abbreviations: CAT, chloramphenicol acetyl transferase; ER, estrogen receptor; ERT, estrogen replacement therapy; SERM, selective estrogen receptor modulator